Affordable for every hospital, clinic and medical practice to have the very best equipment, supplies and service.
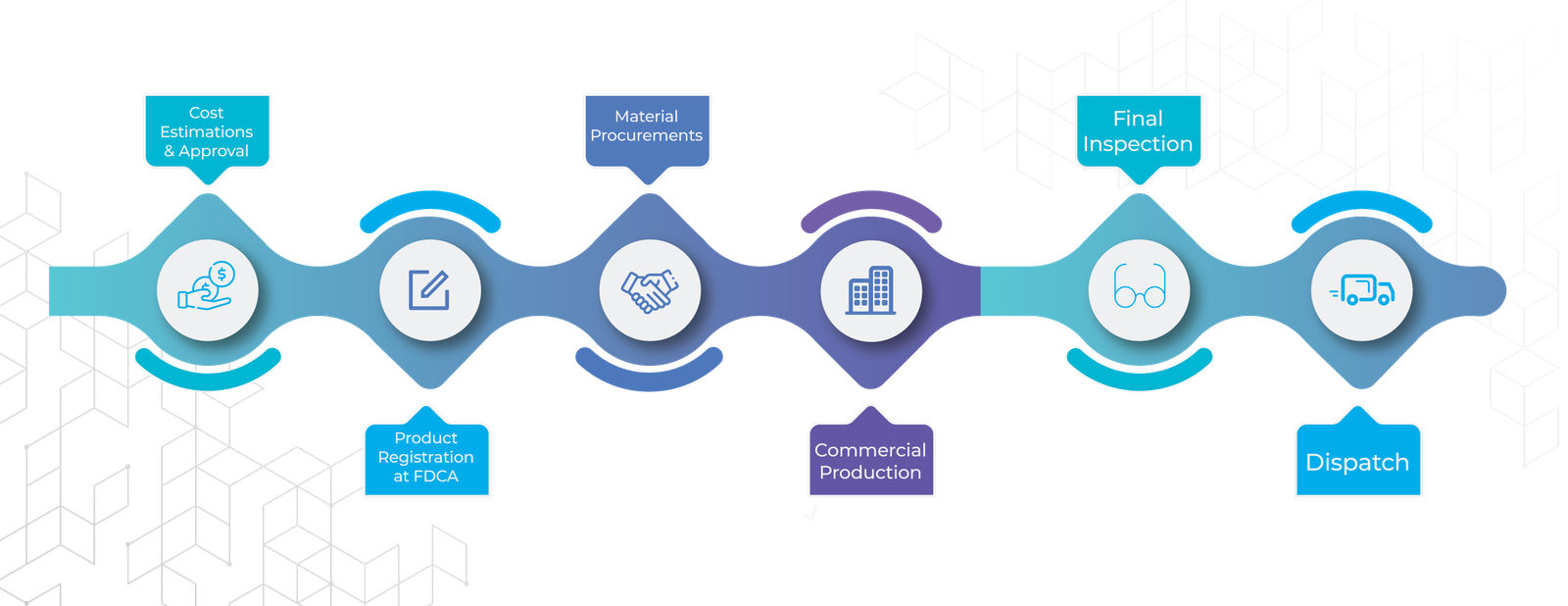
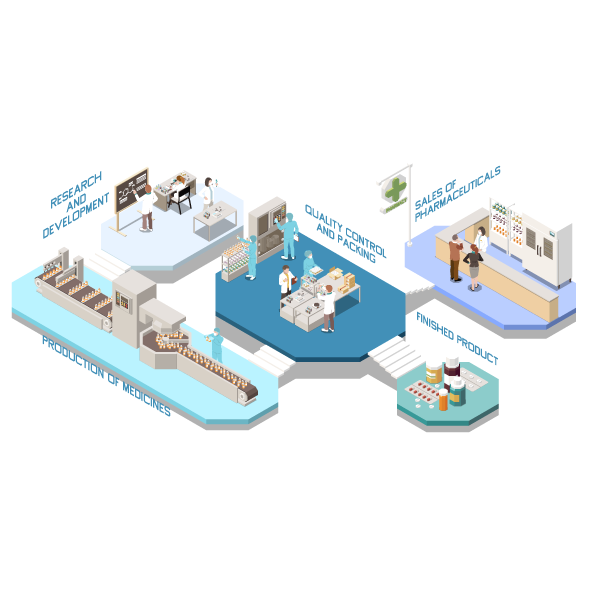
Let us strengthen your business – we have excellent outsourced manufacturers who are equipped with latest machinery and are vividly certified plants World Health Organization, US-FDA, GMP as well as ISO 9001 we believe in helping our clients in every possible way. You can take advantage of our qualified experience and avoid reoccurring threats which affect your business’s ability to expand. We will provide you with best available products, We can also provide you every bit of industrial information that will help you to sky-rocket your revenues, and get ahead of your competitors.
Curensa Healthcare
B/h APMC market, Dhedhal cross road,
Ahmedabad 382220, Gujarat, India
Call : +91 7874471744
Sales: sales@curensahealthcare.com
Enquiry: info@curensahealthcare.com
©2019. Curensa Healthcare . All Rights Reserved.